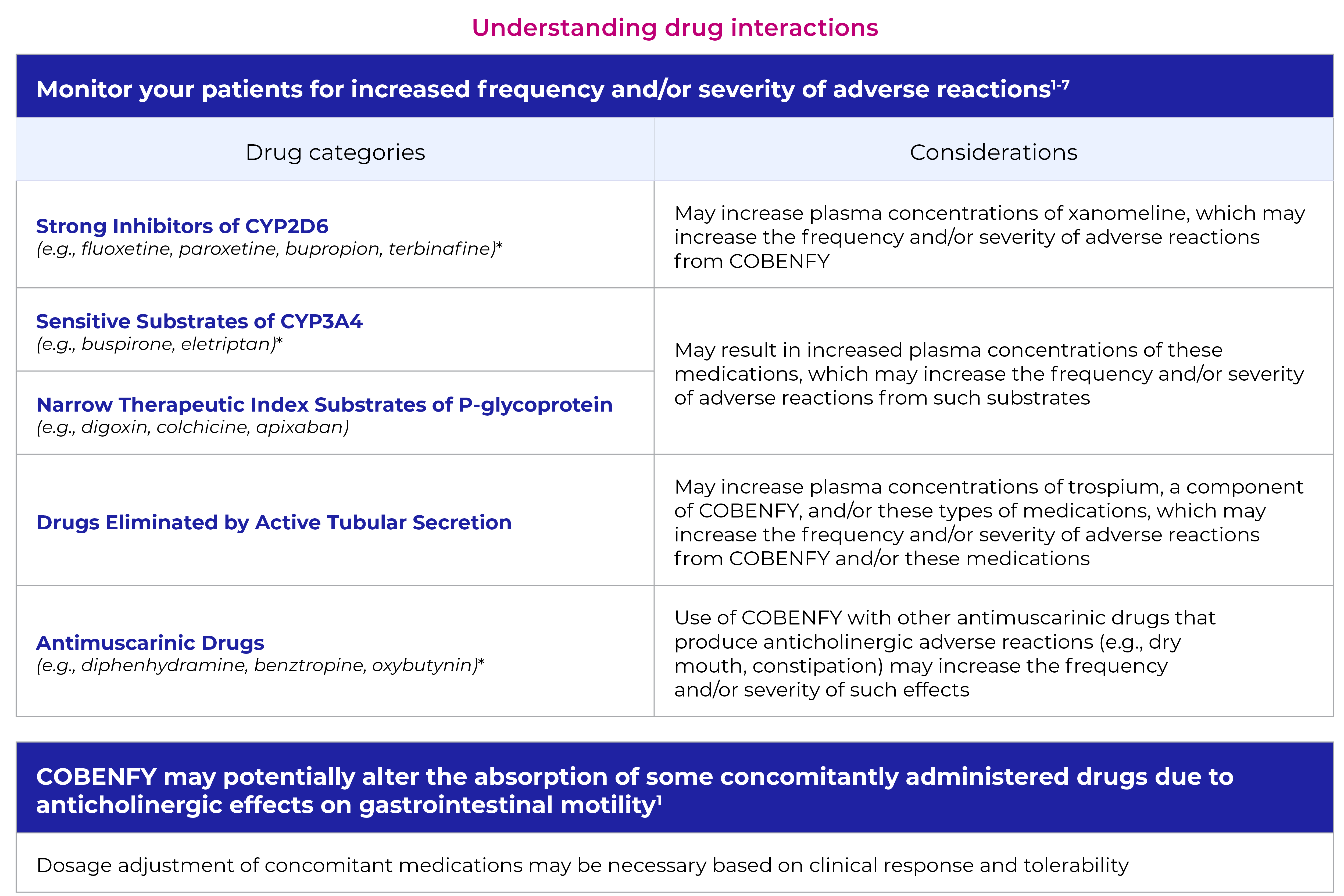
Additional consideration:
Anticholinergics (muscarinic antagonists) and muscarinic agonists may affect the pharmacological activity of one another.
*This is not an exhaustive list and is only used to show clinically relevant examples of medications. For a full list, visit FDA.gov.2








